-
 Overview
Overview
Centre Testing International Group Co., Ltd. (CTI) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
Sustainability
By building a full value chain ESG governance system covering the strategic decision-making level, management execution level and business operation level, it actively practices penetrating management of ESG risk and opportunities, empowering sustainable development across the industry chain.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (CTI) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
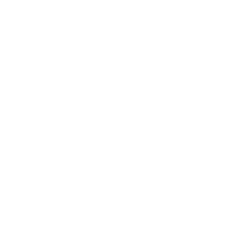
CTI provides you with pharmaceutical instrumentation and computerized system validation services, which can perform GMP validation on warehouses, box equipment, instruments and computerized systems in accordance with the GMP regulations and guidelines of various countries, combined with the needs of users, and has a professional GMP validation team, high-standard testing equipment, and a perfect quality management system.

Equipment Validation
CTI, in accordance with the GMP regulations and guidelines of various countries, JJF, GB and other requirements, combined with the user's actual use of the scope, to provide storage temperature and humidity, refrigerator/incubator/sterilization cabinets and other chamber parameters (temperature, carbon dioxide, pressure, etc.) performance confirmation services to ensure that the warehouse, the chamber in the normal operating methods and process conditions can be sustained.Under normal operating methods and process conditions, we can continuously meet the established acceptance standards.
|
Refrigerator |
Incubator |
Stability Chamber |
Sterilizer |
Cold Storage |
Normal Temperature Storage |
Analytical Instruments and Computerized Systems Validation
CTI provides full validation cycle services (including URS, VP, RA, FS, CS, DQ, IQ, OQ, PQ, VSR, etc.) from validation plan to final system release according to the requirements of GMP regulations and guidelines such as GAMP5 Risk Benchmarking, and in combination with the user's actual needs, in order to prove that the computerized system meets the user's needs and works stably in the long term, and meets the requirements of data integrity. data integrity requirements
|
Type |
Analytical instruments and workstations |
Large laboratory management systems |
|
CSV Service |
Analyst Software for Sciex LCMSMS |
Empower 3 CDS |
|
Easy2Sync Backup Software |
SDS Software for Thermo PCR |
|
|
Vaisala Veriteq CMS- Viewlinc |
Gen5 Data Analyst Software |
|
|
For more information please contact staff... |
||






























 粤公网安备 44030602000441号
粤公网安备 44030602000441号