-
 Overview
Overview
Centre Testing International Group Co., Ltd. (CTI) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
Sustainability
By building a full value chain ESG governance system covering the strategic decision-making level, management execution level and business operation level, it actively practices penetrating management of ESG risk and opportunities, empowering sustainable development across the industry chain.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (CTI) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
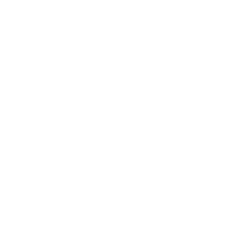
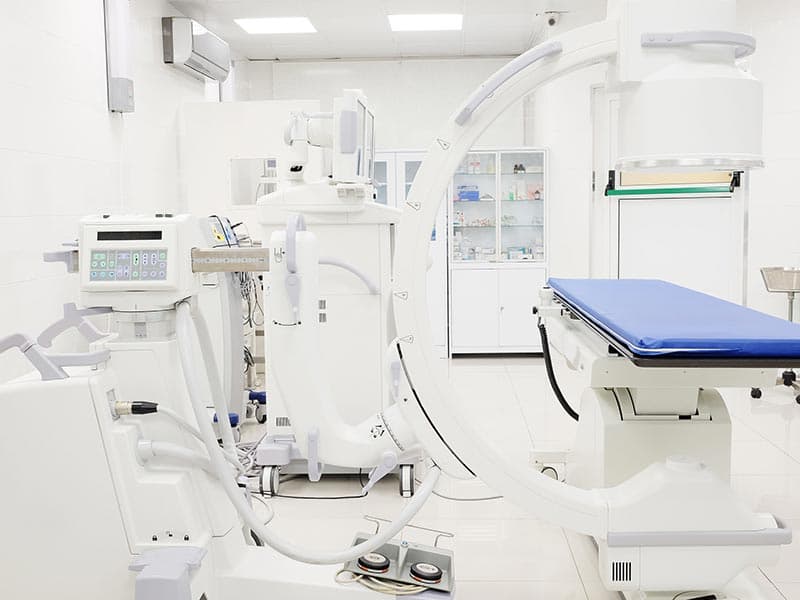
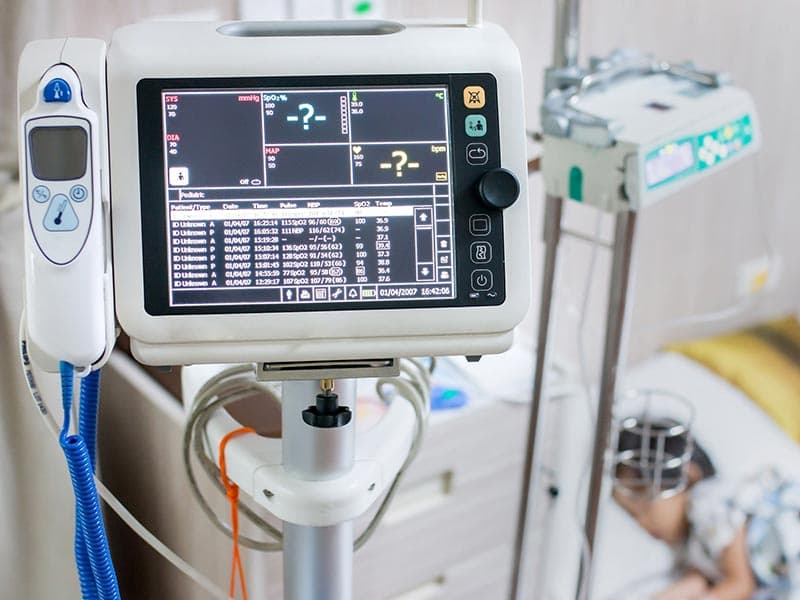
The linear transformer winding of medical electrical equipment needs to have sufficient insulation to prevent dangerous situations that may arise from overheating in internal short circuits. The transformer voltage and frequency doubling test, which evaluates quality and electrical safety characteristics, is one of the most important tests for safety of medical electrical products.

◉ Service Background
Transformer Voltage and Frequency Doubling:The linear transformer winding of medical electrical equipment needs to have sufficient insulation to prevent dangerous situations that may arise from overheating in internal short circuits. For transformer’s winding with rated voltage less than or equal to 500V, or rated frequency less than or equal to 60Hz, 5 times the rated voltage or maximum rated frequency will be will be used, applying a frequency not less than 5 times the rated frequency at both ends of the winding. During the testing process, the insulation should not be broken. The transformer voltage and frequency doubling test, which evaluates quality and electrical safety characteristics, is one of the most important tests for safety of medical electrical products
Medical device testing is the basis for medical devices to enter the global market. If a product is to be exported to the European Union or the United States, then corresponding tests will need to be done to meet the EU or US standards.
◉ Other Services of Medical Devices
- Testing of active medical device
- EMC testing and debugging service
- Reliability Testing
- NMPA registration testing service
- Validation of product life
- Failure mode analysis
- biocompatibility test
- Software evaluation
- Global marketing access
- US agent service
- EU representative service
- Training Service
◉ Medical Device Testing Standards
In Vitro Diagnostic Medical Devices
|
SN |
Title |
International Standard |
National Standard |
|
1 |
Electrical Equipment For Measurement, Control, and Laboratory Use |
IEC/EN 61010-1 |
GB4793.1 |
|
2 |
Safety requirements for electrical equipment for measurement, control and laboratory use –Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical equipment |
IEC/EN 61010-2-101 |
YY 0648 |
|
3 |
Safety requirements for electrical equipment for measurement, control and laboratory use – Part 081: Particular requirements for automatic and semiautomatic laboratory equipment for analysis and other purposes |
IEC/EN 61010-2-081 |
GB4793.9 |
|
4 |
Electrical equipment for measurement, control and laboratory use – EMC requirements - Part 1: General requirements |
IEC/EN 61326-1 |
GB/T 18268.1 |
|
5 |
Electrical equipment for measurement, control and laboratory use – EMC requirements - Part 2-6: Particular requirements - In vitro diagnostic (IVD) medical equipment |
IEC/EN 61326-2-6 |
GB/T18268.26 |
Medical Device Testing Standards List
|
SN |
Title |
International Standard |
National Standard |
|
1 |
Medical safety and electrical essential equipment performance - Part 2-25: of electrocardiographs Particular requirements |
IEC/EN 60601-2-25 |
GB 10793 |
|
2 |
Non-invasive sphygmomanometers - Part 1: Requirements and test methods for non-automated measurement type |
EN ISO /ISO 81060-1 |
|
|
3 |
Non-invasive sphygmomanometers - Part 3: Supplementary requirements for electro-mechanical blood pressure measuring systems |
EN 1060-3 |
|
|
4 |
Medical electrical equipment -- Part 1-1: General requirements for safety - Collateral standard: Safety requirements for medical electrical systems |
IEC/EN 60601-1-1 |
GB 9706.15 |
|
5 |
Medical electrical equipment - Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment |
IEC/EN 60601-2-27 |
GB 9706.25 |
|
7 |
Medical electrical equipment-Part 2-26: Particular requirements for the safety of electroencephalographs |
IEC/EN 60601-2-26:2012 IEC 80601-2-26:2019 |
GB 9706.26 |
|
8 |
Medical safety and electrical essential equipment-Part performance2-56: of clinical Particular thermometers requirements for basic body temperature measurement |
ISO/ EN ISO 80601-2-56 |
|
|
9 |
Non-invasive automated sphygmomanometers |
ANSI/AAMI SP10 |
YY 0670 |
|
10 |
Medical electrical equipment - Part 2-57: Particular requirements for the basic safety and essential performance of non-laser light source equipment intended for therapeutic, diagnostic, monitoring and cosmetic/aesthetic use |
IEC/EN 60601-2-57 |
|
|
11 |
Basic Safety Electrical And Equipment Essential -Performance Part 2-2: Particular Of High Requirements Frequency For Surgical Equipment And High Frequency Surgical Accessories |
IEC/EN/EN IEC 60601-2-2 |
GB 9706.4 |
|
12 |
Medical electrical equipment - Part 1: General requirements for basic safety and essential performance |
ANSI/AAMI ES60601-1 EN /IEC60601-1 |
GB9706.1 |
|
13 |
Medical electrical equipment - Part 2-10 : particular requirements for the safety of nerve and muscle stimulators |
IECC/EN 60601-2-10 |
YY 0607 |
|
14 |
Medical electrical equipment - Part 2-30: Particular requirements for basic safety and essential performance of automated non-invasive sphygmomanometers |
IEC/EN 80601-2-30 |
YY 0667 |
|
15 |
Medical electrical equipment-Part 2-49: Particular requirements for the safety of multifunction patient monitoring equipment |
IEC/EN 60601-2-49/IEC 80601-2-49 |
YY 0668 |
|
16 |
Medical electrical equipment — Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment |
EN ISO 9919 ISO / EN ISO 80601-2-61 |
YY 0784 |
|
18 |
Medical electrical equipment - Part 2: Particular requirements for the safety of electrically operated hospital beds |
IEC/EN 60601-2-52 |
YY 0571 |
|
19 |
Part 2-51: Particular requirements for safety, including essential performance, of recording and analysing single channel and multichannel electrocardiographs |
IEC/EN 60601-2-51 |
YY 0782-2010 |
|
20 |
Diagnostic electrocardiographic devices |
ANSI/AAMI EC11 |
YY 1139 |
|
21 |
Electrocardiographic |
ANSI/AAMI EC13 |
YY1079 |
|
22 |
Part 1-11: General requirements for basic safety and essential performance –Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment |
IEC/EN 60601-1-11 |
|
|
23 |
Performance of compact electrical thermometers (non-predictive and predictive) with maximum device |
EN 12470-3 ASTM E1112 |
|
|
24 |
Performance of electrical thermometers |
EN 12470-4 |
YY 0785 |
|
25 |
infrared ear thermometers (with maximum device) |
EN 12470-5 ASTM E1965-98 |
GB/T 21417.1 |
|
26 |
Part 2-47: Particular requirements for the basic safety and essential performance of ambulatory electrocardiographic systems |
IEC/EN 60601-2-47 AAMI/ANSI/ISO 60601-2-47:2012 |
YY 0885 |
|
27 |
Part 2-22: Particular requirements for basic safety and essential performance of surgical, cosmetic, therapeutic and diagnostic laser equipment |
IEC/EN 60601-2-22 |
GB 9706.20 |
|
28 |
Part 2-41: Particular requirements for the basic safety and essential performance of surgical luminaires and luminaires for diagnosis |
IEC/EN 60601-2-41 |
YY 0627 |
|
29 |
Part 2-46: Particular requirements for the basic safety and essential performance of operating tables |
IEC/EN 60601-2-46 |
YY 0570 |
|
30 |
ECG trunk cables and patient leadwires |
AAMI ANSI EC53 |
YY 0828 |
|
31 |
Part performance 2-18: Particular of endoscopic requirements equipment |
IEC/EN 60601-2-18 |
GB9706.19 |
|
32 |
Medical electrical equipment-Part 1-2:General requirements for safety Collateral standard: Electromagnetic compatibility-Requirements and tests |
IEC/EN 60601-1-2 |
YY 0505 |
|
37 |
Medical electrical equipment- Part 1-8: General requirements for safety Collateral standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems |
IEC/EN 60601-1-8 |
YY0709 |
|
38 |
Safety of laser products —Part 1: Equipment classification and requirements |
IEC/EN 60825-1 AS/NZS 2211.1 |
GB 7247.1 |
|
39 |
Photobiological safety of lamps and lamp systems |
IEC /EN62471 AS/NZS IEC 62471 IEC IEC/TR 62778 IEC/TR 62471-2 |
GB/T 20145 |
|
40 |
RESPIRATORY THERAPY EQUIPMENT –Part 1: Nebulizing systems and their components |
EN13544-1 |
YY 0109 |
|
41 |
Medical electrical equipment –Part 2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers |
IEC/EN 60601-2-24 |
GB 9706.27 |
|
42 |
Medical electrical equipment - Part 2-34: Particular requirements for the basic safety and essential performance of invasive blood pressure monitoring equipment |
IEC/EN 60601-2-34 |
YY0783 |
|
43 |
Medical electrical equipment –Part 2-60: Particular requirements for the basic safety and essential performance of dental equipment |
IEC/EN 80601-2-60 |
|
|
44 |
Medical devices IEC devices 62366-1: - Part 2015 1: IEC Application 62366: 2007+A1:2014 of usability engineering |
IEC/EN 62366-1 |
YY/T 1474 |
|
45 |
Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability |
IEC/EN 60601-1-6 |
|
|
46 |
Medical electrical equipment -Part 2:Particular requirements for safety of baby incubators |
IEC/EN 60601-2-19
|
GB 11243 |
|
47 |
Medical electrical equipment Part 1-9: General requirements for basic safety and essential performance– Collateral Standard: Requirements for environmentally conscious design |
IEC/EN 60601-1-9 |
|
|
48 |
Clinical electronic thermometer |
|
GB/T21416 |
|
49 |
Medical device software — Software life cycle processes |
IEC62304 |
YY/T 0664 |
|
50 |
Medical suction equipment Part 1: Electrically powered suction equipment — Safety requirements |
ISO10079-1 |
|
|
51 |
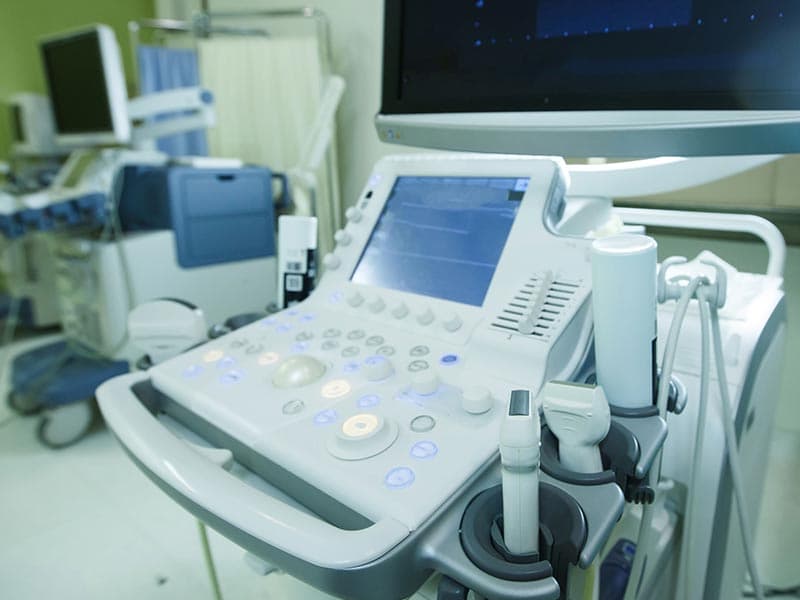
Medical electrical equipment — Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment |
EN 60601-2-37 |
GB 9706.9-2008 GB10152-2009 |
|
52 |
Standard means for the reporting of the acoustic output of medical diagnostic ultrasonic equipment |
IEC 61157 |
GB/T16846 UD2 UD3 |
|
53 |
Ultrasonics dental descaler systems; measurement and declaration of the output characteristics |
IEC 61205 |
YY/T 0751 |
|
54 |
ultrasonic Doppler fetal heartbeat detector |
|
YY 0448 |
|
55 |
ultrasonic Doppler fetal monitor |
|
YY/T0449 |
|
56 |
Medical electrical equipment — Part 2-5: Particular requirements for the basic safety and essential performance of ultrasonic physiotherapy equipment |
IEC/EN 60601-2-5 |
YY/T1090 GB9706.7 |
|
57 |
ultrasonic-Hydrophone Part 1: Measurement and characterization of medical ultrasonic fields to 40MHZ |
IEC62127-1 |
YY/T 0865.1 |
◉ Applicable Products
Medical devices (including: thermometers, oximeters, sphygmomanometers, fetal heartbeat detector, electrocardiographs Color ultrasound system, X-ray machines, etc.)
◉ Sample Requirements
Complete and fully-functional samples. For more specific details, please contact CTI online customer service.
◉ Our Edge
|
|
|
|
◉ FAQ
How long is the lead-time for medical device testing?
Actual lead-times are based on the product and standards, please contact CTI customer service for more details.
What test method does CTI use for medical device testing?
The applied test and standards are carried out according to the export requirements or to the clients’ needs.
What is CTI’s retention period of samples?
Sample Return



















 粤公网安备 44030602000441号
粤公网安备 44030602000441号