-
 Overview
Overview
Centre Testing International Group Co., Ltd. (CTI) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
Sustainability
By building a full value chain ESG governance system covering the strategic decision-making level, management execution level and business operation level, it actively practices penetrating management of ESG risk and opportunities, empowering sustainable development across the industry chain.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (CTI) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
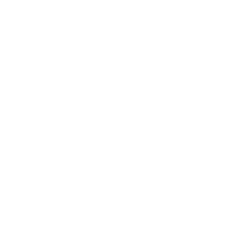
CTI has the qualification of cosmetics registration and filing inspection agency, and can undertake non-special use cosmetics filing inspection work, including microbes, heavy metals, dioxane, free formaldehyde, asbestos and other prescribed inspection items, and can carry out the test of diethylene glycol, hydroquinone, phenol and other risk substances according to the formula and characteristics of the product.

◉ Business Challenges
The quality and safety of cosmetics is a particular concern for all stakeholders including brands, regulators, and consumers.
Safety indicators are mandatory for the development of high-quality products and can be difficult to ascertain without independent verification.
Raw materials of cosmetics continue to be a critical concern due to their impact on the overall safety and compliance of cosmetic products sold both domestically and abroad.
As a leading solutions provider, CTI's core business is the independent verification of product quality and safety to meet the demands of both consumers and regulators.
◉ Service Content
With the changes in cosmetics regulatory policies, the filing review requirements have become more stringent. The National Medical Products Administration issued a notice on “Adjusting the Administration of Cosmetics Registration and Filing (No. 10, 2013)" which stipulates that before a company's product can go on sale, a "product inspection report" must be provided by the "Cosmetics Registration and Filing Inspection and Testing Agency" approved by National Medical Products Administration.
CTI holds this qualification as a cosmetics registration and filing inspection agency, and can undertake non-special use cosmetics filing inspection work, including microbes, heavy metals, dioxane, free formaldehyde, asbestos and other prescribed inspection items. According to the formula and characteristics of the product, we can also carry out the test of diethylene glycol, hydroquinone, phenol and other risky substances present in final products, raw materials, or packaging. We can also provide enterprises with technical consulting services related to cosmetic safety risk assessment and filing.
|
Testing item |
Test method |
Test qualification |
Sample amount |
Working day |
|
9 items of non-special cosmetics filing inspection routine: total number of colonies, total number of molds and yeasts, heat-resistant coliforms, Staphylococcus aureus, Pseudomonas aeruginosa, mercury, lead, arsenic, cadmium |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
80g/ml Guarantee 3 or more independent packaging |
7~9 |
|
Methanol |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
20g/ml |
7~9 |
|
Dioxane |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
10g/ml |
7~9 |
|
Asbestos |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
10g |
7~9 |
|
Free formaldehyde |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
30g/ml |
7~9 |
|
Chemical UV filters |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
10~20g/ml |
7~9 |
|
pH Value |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
50g/ml |
7~9 |
|
Alpha-hydroxy acid (10 items) |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
20g/ml |
7~9 |
|
Anti-dangdruff agent |
“Safety and Technical Standards for Cosmetics " (2015 Edition) |
CMA、CNAS |
10g/ml |
7~9 |
◉ Our Advantages
Recognition: Our expertise and experience ensure your products meet or exceed expectations. We have served nearly 100,000 customers, including more than 100 of the world's top 500 companies, and have issued more than 3 million test and certification reports.
Coverage: We provide you with the most complete capabilities as possible, by owning and operating more than 150 laboratories and 90 hubs across a variety of industries.
Expertise: Our research and development have led to the formulation of more than 540 national and industry standards, and we have secured more than 470 national patents.
Quality: To ensure efficiency, quality, and accuracy, we have implemented an advanced global laboratory information management system to align and optimize the operation of each service line; ensuring that any CTI report produced is held to the highest levels of accuracy and integrity.




















 粤公网安备 44030602000441号
粤公网安备 44030602000441号