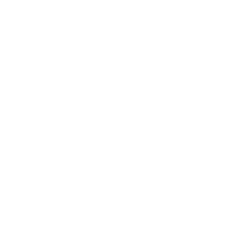
We provide elemental impurity analysis for raw and auxiliary materials and finished products. Analytical method development meets the requirements of ICH Q14 guidelines; analytical method validation meets the requirements of ICHQ2(R2) guidelines, USP<1225>, <233> General Principles, ChP<9101> General Principles, and other pharmacopoeias or guidelines.
-
 Overview
Overview
Centre Testing International Group Co., Ltd. (CTI) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
Sustainability
By building a full value chain ESG governance system covering the strategic decision-making level, management execution level and business operation level, it actively practices penetrating management of ESG risk and opportunities, empowering sustainable development across the industry chain.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (CTI) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
Home > About CTI > 信息列表 > > News Detail
Analysis of Elemental impurities In Raw and Auxiliary Drugs and Finished Products
Related recommendations
Media Inquiries
- 400-6788-333
- mkd@cti-cert.com
















 粤公网安备 44030602000441号
粤公网安备 44030602000441号